Feverish
As another flu season begins, a larger question looms: how can folks gain easier access to a potentially more effective flu vaccine not made with chicken egg-based technology?
In 1925, in an address to American Society of Newspaper Editors, President Calvin Coolidge, in a speech entitled, “The Press under a Free Government,” said: “The chief business of the American people is business.” It is an often cited if misquoted phrase.
The people, Coolidge continued in his talk, “are profoundly concerned with producing, buying, selling, investing and prospering in the world. I am strongly of the opinion that the great majority of people will always find these the moving impulses of our life.”
For Coolidge, there was a natural relationship between business and the news media: “A press which maintains an intimate touch with the business currents of the nation is likely to be more reliable than it would be if it were a stranger to these influences.”
Sixteen years later, in 1941, in his inaugural address, President Franklin D. Roosevelt offered a different vision of the business of the American people, what he described as the four freedoms: freedom of speech, freedom of worship, freedom from want, and freedom from fear, made famous in a series of paintings by Norman Rockwell.
The four freedoms, Roosevelt said, were the foundation of the benefits of democracy, which included economic opportunity, employment, social security and the promise of adequate health care.
In 2018, the role of the news media has morphed to a point where corporate ownership and consolidation threatens to overwhelm the role of the free press in a democratic government. The question is not about protecting business interests or protecting freedoms but the willingness to call out blatant lying by the President.
When the President calls the news media the enemy of the people because it reports with critical scrutiny on his actions, when the government of Saudi Arabia can allegedly murder an American journalist in their consulate in Istanbul, Turkey, what is the proper response?
In the last week, in a terrorist attack, bombs were mailed to former Presidents, a former Vice President, elected Senators and Congresswomen, and CNN, among others, all who had been targeted repeatedly by derisive invective and taunts by President Trump at campaign rallies.
The arrest of the alleged bomber was followed by an attack on a Pittsburgh synagogue by a gunman who announced he wanted to kill Jews. Closer to home, neo-Nazis have targeted Sam Bell, the new elected state senator from Providence, and his wife with vile, hate-filled rhetoric, for being Jewish and bi-sexual.
The looming question for the news media, and for every citizen, is how best to respond to such outrages: is it business as usual?
A further dilemma, particularly for the news media, is how to respond to the blatant lies by the President, his supporters and his enablers.
For the record, there is no planned tax cut for middle-class Americans before the election, in large part because Congress is not in session. The stock market did not open the next day after the terrorist attack on Sept. 11, 2001. The Republicans have not supported protections against pre-existing conditions but rather sought to eliminate them in their repeated efforts to repeal Obamacare.
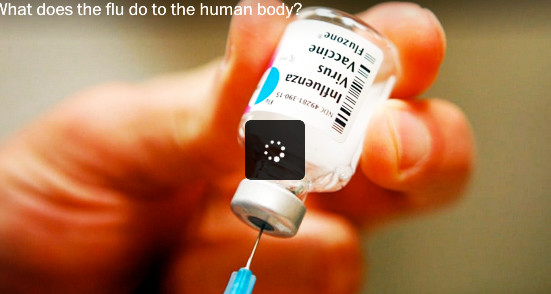
PROVIDENCE – In movies and TV shows, it is called product placement or embedded marketing: consumer products that appear to be innocuously placed within the hand of the character, such as a soda can or a candy bar, or the make of the car being driven by the star. But what is it called when the apparently inadvertent product placement is a vial of flu vaccine in a prominent feature story in The Washington Post?
In its recent story by reporter Lena Sun, “Drop in adult flu vaccinations may be factor in last season’s record-breaking deaths, illnesses,” The Washington Post used as its illustration a video that began with an identifiable image of a vial of Sanofi Pasteur’s Fluzone influenza vaccine.
The story itself began: “Fewer than 4 out of 10 adults in the United States got flu shots last winter, the lowest rate in seven seasons and one likely reason that the 2017-2018 season was the deadliest in decades.”
As reported in a recent story by the Associated Press, an estimated 80,000 Americans died of flu and its complications last winter, the disease’s highest death toll in at least four decades, according to the Centers for Disease Control and Prevention.
Sun wrote: “Flu vaccination is the main way to prevent sickness and death caused by flu. But last season, vaccination coverage among adults was 37.1 percent, a decrease of 6.2 percentage points from the previous season. That’s the lowest rate for adults 18 and older since 2010-2011.”
What is the cause of that low rate of vaccination? The worry, expressed by experts in Sun’s story, is that anti-vaxxers have contributed to the fact that many adults do not get vaccinated against the flu, despite the overwhelming clinical evidence about the effectiveness of flu vaccines.
In a world where anti-science rhetoric has become a staple of so-called “populist” movements here in the U.S. and in Europe, concerns are being voiced not just about the flu but the outbreak of measles: in Europe in 2018, with more than 40,000 cases reported. Here in the U.S., there have been 142 cases reported in 25 states as of Oct. 6, 2018, according to the CDC.
The disliked reality is that that travelers with measles continue to bring the disease into the U.S., which perhaps can be seen as the true toxic caravan threat. For instance, the New York City Department of Health recently reported an outbreak of measles in the Orthodox Jewish Community in Williamsburg, Brooklyn, spread by an unvaccinated child who contracted the disease during a recent visit to Israel. Another seven measles cases were confirmed in New York State residents outside of New York City, five of which were acquired during a visit to Israel.
[Instead of attemptiong to separate children from parents when asylum seekers come to the U.S., perhaps the Department of Homeland Security should provide free vaccinations for measles, mumps, rubella and flu.]
The scientific subtext
But, even among the most fervent advocates of flu vaccinations, there is an ongoing debate about whether the current technology used to manufacture flu vaccines, the fertilized chicken egg-based process, is the most efficacious method.
As reported by ConvergenceRI in February of 2018: “With the severity of this year’s flu season has come questions about the effectiveness of the current flu vaccine manufactured using egg-based technology, in particular, concerns about whether the drift of the flu virus during the manufacturing process of the vaccine translated into less effective protection against the predominant flu virus strain in circulation, H3N2.”
[See link to ConvergenceRI story below, “Achoo: the flu season reveals gaps in public protection.”]
The story continued: “Recent analyses have estimated that the flu shot manufactured through egg-based technology is only 25 percent effective against the H3N2 flu strains circulating this season, according to the Centers for Disease Control and Prevention. In turn, the public messaging about the lack of effectiveness about the flu vaccine may have contributed to some consumers deciding not to get a flu shot this year, which many public health practitioners have warned is a poor choice.”
Further, the ConvergenceRI story reported: “As Helen Branswell of STAT reported in her Feb. 6, 2018, story, “Some flu vaccines may work better than others – but guidance to the public is scant. Last fall some people in the know about influenza science got picky when it came time to get their flu shots.”
The ConvergenceRI story continued, quoting Branswell’s STAT story: “They didn’t want to roll up their sleeve for any old vaccine [offered] at their doctor’s office or workplace clinic. Instead, those in the know sought out specific products, including Flublok, an FDA-approved vaccine made through recombinant technology and not from egg-based technology.”
Fighting flu on numerous fronts
There are some common-sense approaches to flu prevention. Cover your nose and mouth when you sneeze, to avoid spreading germs. Avoid shaking hands during flu season. Regularly wash your hands with soap when you return home from the outside and before you sit down to eat.
On the scientific front line, there have been, in the last few years, two alternative methods of manufacturing flu vaccine which have received FDA approval:
• In 2015, the recombinant DNA approach pioneered by Protein Sciences of Meriden, Conn., for its product, Flublok, received approval for us by adults 18 years of age or older. Flublok Quadrivalent vaccine has been proven in a randomized controlled trial to prevent more cases of influenza in adults 50 years and older compared with a standard-dose quadrivalent inactivated influenza vaccine. [In 2017, Protein Sciences was purchased by Sanofi Pasteur for $750 million; Sanofi Pasteur is the world’s largest vaccine manufacturer.].
• In 2016, the cell culture-based approach pioneered by Seqiris, at its Holly Springs, N.C., facility, for its product, Flucelvax, received approval. Seqiris is a division of CSL, which was created through the merger with Norvartis Influenza Vaccines in 2015, making it the second largest influenza company in the world.
[To manufacture its flu vaccines, Flublok uses insect recombinant DNA; Flucelvax uses animal kidney cells in liquid culture.]
While the Centers for Disease Control and Prevention does not offer any recommendations about the different technologies employed to make flu vaccines, the pricing policies for flu shots and public health bulk-buying programs for flu shots tend to make for a kind of de facto public health policy decision-making.
Those decisions are also being influenced by what huge health corporations such as CVS choose to market as their go-to flu vaccines, how much health insurers will pay for flu vaccines, and willingness of big box stores to invest in offering higher cost [and more effective] flu shots; “free” flu shots are often promoted as a way to lure customers to shop at their stores.
And, of course, whether consumers are being given good enough information from health care providers to ask not just for a flu shot, but to ask for the more effective flu shot.
The power of branding
The placement of the image of Sanofi’s Fluzone in a video about flu vaccines illustrates what can be described, perhaps, as part of a larger, unreported stealth story about the public health choices being made around the purchases of flu vaccines, in which Sanofi is competing not just against other flu vaccine manufacturers but also its own subsidiary.
One Sanofi product, Fluzone, made from egg-based technology, will produce about 70 million doses for sale in the U.S. market, according to company officials. By comparison, roughly 1 million doses of Flublok will be manufactured for the U.S. market, according to sources.
In terms of pricing comparisons, apples to apples, Fluzone quadrivalent vaccine is sold to pharmacies at a wholesale price roughly between $16 and $19 a vial, while the wholesale price for Flublok quadrivalent is roughly three times that, at approximately $46 a vial, according a Sanofi communications spokeswoman. [The retail price is often double the wholesale price, according to one pharmacist – $40 for Fluzone, $80 for Flublok.]
More than the economic dynamics of supply and demand, the question that needs to be asked is: Is Sanofi preserving its market dominance in the flu vaccine marketplace and its egg-based technoilogy by limiting the marketing and pricing strategy for Flublok?
Translated, the playing field appears to be tilted. And, cost becomes a critical factor when public health agencies such as the R.I. Department of Health make decisions about which flu vaccine to purchase: Sanofi’s Fluzone or Sanofi’s Flublok.
“Flublok was not purchased by the R.I. Department of Health for the 2018-2019 flu season,” explained Joseph Wendelken, communications spokesman with the agency. “The CDC does not make product recommendations among the vaccines that are available [beyond age indications].”
Because there was no specific recommendation for it, Wendelken continued, “and because it costs $46 a dose, as opposed to $13 a dose for standard quadrivalent, we purchased other products.”
Additionally, Wendelken said, “We have been offering Fluzone High Dose for the past three or four seasons and the public has become accustomed to this vaccine. In many cases, people request it specifically.”
Wendelken offered further clarification about the agency’s decision: “The CDC did not make a recommendation about people who are 65 years of age and older and [which] quadrivalent [flu vaccines to use],” he said. “It is a decision that health care providers make with their patients.”
Of course, it helps if patients know to ask about Flublok and also know where it is available in Rhode Island, particularly if you are over 50 years of age.
The word from Sanofi
ConvergenceRI reached out to Sanofi Pasteur to get a better understanding of the corporation’s market outreach strategy, asking: how does Sanofi Pasteur manage the messaging so that you are not in competition with two different flu vaccine products, targeted at similar markets?
Here is how Heather Levis Guzzi, director of U.S. product communications for Sanofi, responded:
“Sanofi Pasteur’s portfolio includes two vaccines that are proven to help prevent more flu in older adults, compared to standard-dose flu vaccines – Fluzone High-Dose vaccine and Flublok Quadrivalent vaccine,” Guzzi said.
“Fluzone High-Dose vaccine is the first and only flu vaccine shown to have superior efficacy [24.2 percent more protection] against the flu in adults 65 years of age and older, compared to Fluzone vaccine, a standard-dose inactivated flu vaccine,” Guzzi continued.
The Flublok Quadrivalent vaccine, Guzzi explained further, “is approved for use in people 18 years of age and older. A clinical study of adults 50 years of age older, which was published in The New England Journal of Medicine [in 2017], showed that Flublok Quadrivalent vaccine significantly reduced flu disease compared to a standard-dose quadrivalent inactivated flu vaccine.
Further, Guzzi said: “Flublok Quadrivalent vaccine demonstrated 30 percent more protection from PCR [polymerase chain reaction analysis]-confirmed flu caused by any viral type/subtype, and 43 percent more protection in preventing culture-confirmed flu, compared to a standard-dose quadrivalent inactivated flu vaccine.”
While Guzzi explained the potential benefits of each different flu vaccine product, she did not appear to answer the broader question about how Sanofi Pasteur, the world’s largest manufacturer of flu vaccines, managed two competing products in the same market.
The patient’s view
Since ConvergenceRI first wrote about Protein Sciences and its recombinant flu vaccine in 2015, Flublok, a reader in Dallas, Texas, has become a strong advocate, in part because of her high sensitivity to many medications. The reader has since been recommending Flublok to all of her friends, and reported that she has been flu-free since she began being vaccinated with Flublok.
As ConvergenceRI reported in 2015: Flublok, which Protein Sciences calls the world’s first licensed influenza vaccine that is manufactured using recombinant DNA technology, is approved for use for anyone over 18 years of age.
It is marketed as being a highly pure product: it contains no egg protein, gelatin, thimersal [a mercury derivative], latex, gluten, antibiotics, formaldehyde or influenza virus, according to Protein Sciences.
And, it’s highly effective, according to Protein Sciences. A recent clinical study of Flubok involving approximately 9,000 adults 50 years or older showed that Flublok recipients were 31 percent less likely to develop laboratory-confirmed influenza than people who received a traditional, egg-based vaccine.
Unlike the traditional 70-year-old egg-based technology that is used to produce most flu vaccines, Protein Sciences uses recombinant technology to produce large quantities of the protein that is the active ingredient in flu vaccines.
This year, the reader wrote to ConvergenceRI describing the difficulty she had encountered in locating a pharmacy that carried Flublok. Finally, she was able to locate a pharmacy that was able to purchase Flublok, which she said her health insurance [Medicare] covered in full, at a retail cost of $75.
In an election year, when alleged voter suppression is a big story, the reader railed against what she called a potential case of “drug suppression,” saying that ConvergenceRI should do an investigative story about it – not just with Flublok but with other drugs, too.
ConvergenceRI gets vaccinated with Flublok
In past years, ConvergenceRI has been frustrated in trying to get a flu shot using Flublok. Local pharmacies said they didn’t carry it; his primary care physician said she was unaware of it; communications spokespeople at health insurers said that they were unfamiliar with it.
This year, thanks to a source, ConvergenceRI was provided with link to a locator that included retail pharmacies and medical practices in the region that offered Flublok. [See link below.]
ConvergenceRI went to a nearby pharmacy and, without an appointment, received the Flublok quadrivalent flu vaccine. The retail cost would have been $80, according to the pharmacist, but it was covered in full under ConvergenceRI’s health insurance coverage.
The pharmacist, a graduate of the URI College of Pharmacy, had used Flublok last year for himself and been flu-free, he reported.
He reported that there was increasing interest in Flublok, because it was egg-free, for people with egg allergies, and it was also becoming popular with vegans. The pharmacist also said that one of his colleagues in nearby pharmacy had also ordered Flublok this year.
The moral of the story
A potential advantage of a recombinant DNA flu vaccine such as Flublok is that it can serve as a more nimble approach to combating different flu strains as they evolve and morph each year, compared to egg-based vaccine manufacturing.
In 2018, the 100-year anniversary of the 1918 flu pandemic that killed millions around the world, the biggest fear that many working in the field of public health is the potential of a new pandemic sweeping the globe, caused by the H7N9 flu virus.
However, the question raised by a reader of ConvergenceRI is apt: how can the world’s largest manufacturer of flu vaccines, Sanofi Pasteur, compete against itself in the market place, with two separate products? Does that become a case of drug suppression?
Imagine, for instance, if the image contained in the video used to illustrate the recent Washington Post story had been a vial of Flublok, and not Fluzone?