IlluminOss expands its bone fracture repair system in U.S. commercial market
Minimally invasive system can now be used with mature patients to repair fractures in fragile arm bones by itself, or combined in collaboration with other surgical hardware, such as plates, screws and plates
BREAKING NEWS
EAST PROVIDENCE – On Tuesday, Feb. 26, IlluminOss Medical, Inc., announced that it was launching the commercial availability in the U.S. market for its innovative, minimally invasive bone fracture repair system, targeting fragility fractures of large arm bones [the humerus, radius and ulna] in an aging population, resulting in a potential 10-fold increase in its market, according to Jeff Bailey, the CEO of the privately held, commercial stage medical device firm.
“Entry into the multi-billion dollar U.S. trauma market marks a significant milestone for our company,” Bailey said. The launch is being timed to coincide with the annual meeting of the American Academy of Orthopaedic Surgeons in Las Vegas, from March 12-16, according to Bailey, where a presentation of data on the innovative system is planned for March 13.
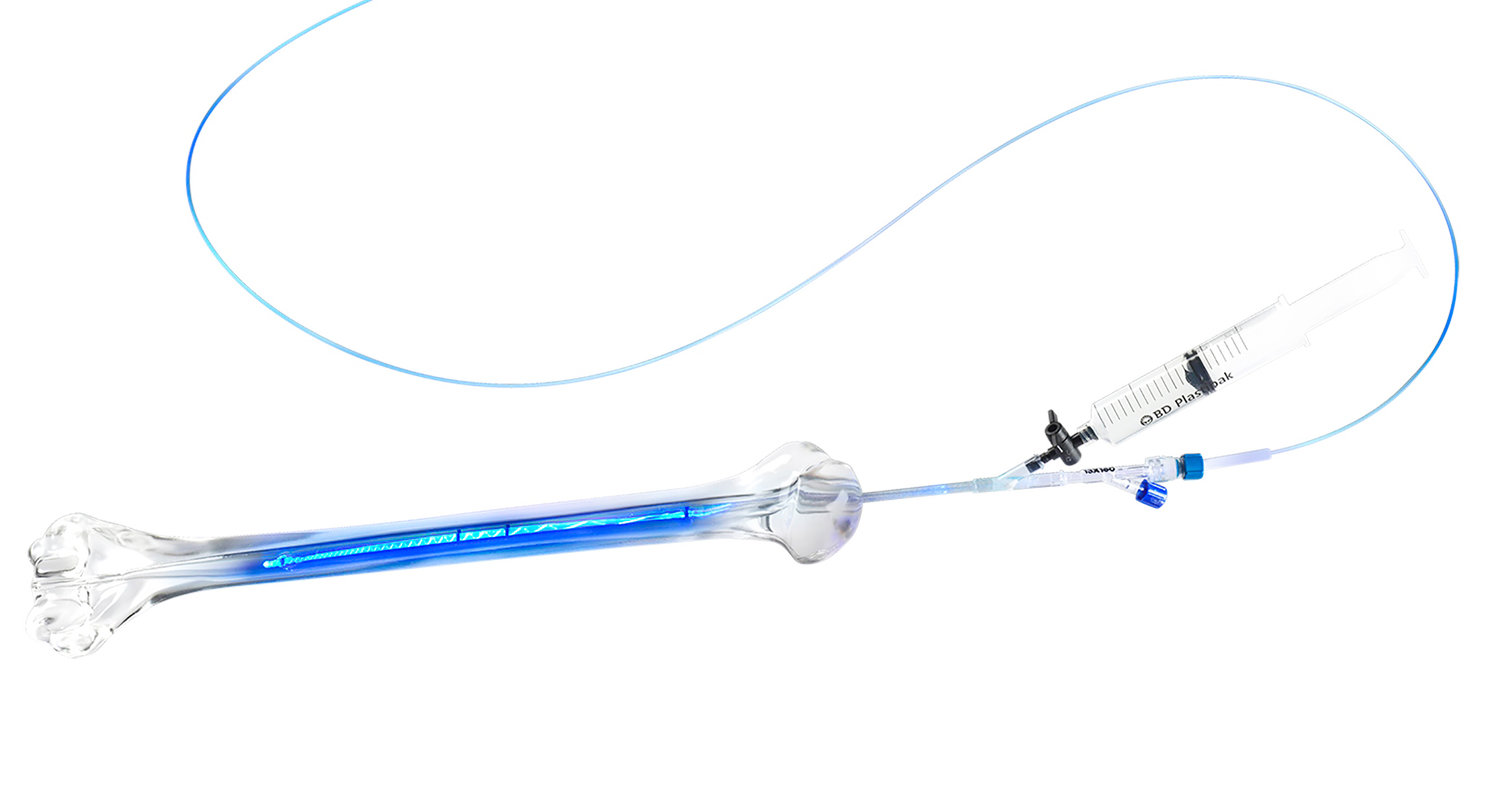
The IlluminOss bone fracture repair system utilizes an expandable balloon implant that is delivered in a minimally invasive manner through a small incision, as described in the news release. When the implant is infused with mononer, it conforms to the patient’s intramedullary bone canal. A visible blue light rapidly then rapidly cures the implant, stabilizing the fracture.
The IlluminOss system received its initial de novo FDA clearance in December of 2017 for fractures associated with metastatic bone. [See link below to ConvergenceRI story, “IlluminOss granted FDA OK for its innovative bone stabilization system.”]
The de novo approval was granted following U.S. clinical trials that began in 2014 and were completed in 2016. Additional clearance was received in August of 2018 for traumatic and fragility fractures. The IlluminOss system has been commercially available in international markets and has been in clinical use since 2010.
“IlluminOss provides an exciting new option in fracture repair for difficult-to-treat osteoporotic or other patients with compromised bone, and is a much-needed addition to conventional treatment with nails, plates and screws,” said Dr. Mark Goodman, University of Pittsburgh Medical Center, as quoted in the news release.
Goodman, who had been a participant in the clinical trials for the IlluminOss bone repair system, called the IlluminOss system a “game-changer.” “The fact that this unique technology was designed specifically for use in patients with compromised bone enables surgeons to create a conforming implant for each individual patient and is a game-changer in fracture repair,” he said.
Among the findings from the clinical trials, surgeons who have utilized the innovative IlluminOss system have reported: reduced hospital stays, shorter procedure times, less post-operative pain, less use of pain medications and patients and a faster return by patients to their daily living activities.
One of the advantages of what the firm called its “revolutionary” procedure is that it uses a small percutaneous surgical approach, providing patients and clinical providers with a fast, patient-specific method of orthopedic bone stabilization, according to the news release.
East Providence footprint
In an interview with ConvergenceRI, Bailey said that the company has no plans to relocate from its headquarters in East Providence. “Our footprint is in East Providence,” he said. “We are happy with our capacity, our clean room environment, our on-site, high-functioning manufacturing.”
In turn, Bailey praised the leadership of Robert Rabiner, the chief technology officer at IlluminOss, whom he called the backbone of the company.
In ramping up for the launch in the U.S. commercial market, Bailey said that the firm had hired two more salespeople, bringing the total to four, taking a slow but steady approach to expanding its market reach.
The IlluminOss product, Bailey continued, can be used instead of the traditional hardware of screws, plates and nails in surgical repair. “It can also be used in combination with plates, screws and nails,” he explained, providing surgeons with another treatment option with fragile bones.
Future plans
Now that the IlluminOss bone fracture repair system has been approved for use in fragile large arm bones in the upper extremities of the body, Bailey said that, in time, the firm’s innovative approach to bone fracture repair may be expanded for use in the lower extremities, which will involve more clinical studies and more regulatory approval.