Do we owe our souls to the company store?
The next episode of the hamster wheel in health care kicked in on Jan. 1, when the new annual fees for co-pays and co-insurance began again, requiring patients to pay more
Researchers are exploring how the lingering effects of COVID may be linked to the physiological toll of the body’s immune system and its response to the infection, including making it harder for the brain to get the nutrients it needs, as well as its ability to fight off harmful pathogens.
Until there is more research conducted on the long-term impacts of COVID on the body’s organs, including the brain, reporters and commentators and politicians should refrain from categorizing any cases of COVID as “mild.”
PROVIDENCE – For those of us who have health insurance plans with some amount of co-insurance and co-pays attached to the delivery of care [which is just about everybody], Jan. 1, 2022, arrived with the dread of knowing that the cost of health care has just gone up – and with it, a new season of self-rationing, a kind of risk assessment that covers all transactions across the health care landscape. We have learned how to cope, to scrimp, to postpone, and to avoid – survival skills for those of us with chronic health conditions and the need to pay attention to monthly budgets.
The health care delivery enterprise may be crashing and burning all around us, and the lines to get tested for COVID may stretch around the city block, but there is a certainty attached to my next physical therapy appointment this week, that it will come with a $25 co-pay. Physical therapy is the best possible treatment recommended to manage my health condition, according to a consensus from all my health care providers, so co-pays, here I come.
What purpose co-pays and co-insurance serve in the health care and health insurance market is unclear, even when I have asked health insurance executives and health care providers about what such payments accomplish, in terms of the how it impacts and improves the bottom line.
Providers dislike it, because of the busy work it creates. Patients dislike it, because it seems to be such an arbitrary amount. Policy analysts dislike it, because it can get in the way of accessing care, often resulting in postponed care that can lead to more complications down the road. To paraphrase Langston Hughes, “What happens to a health care appointment deferred?”
Policy debates
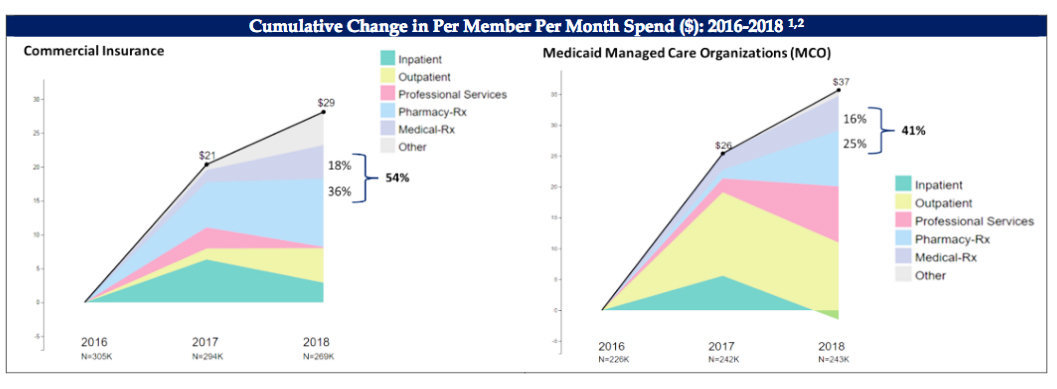
High pharmacy costs are the major driver of increased health costs in Rhode Island, according to the most recent data analyses conducted by a consortium of groups. [See link below to ConvergenceRI story, “Prescription drugs, not utilization, are driving high health costs in RI.”]
The increase in health costs is not being driven by utilization; it is not being driven by care for high-risk, complex patients. And, it is not being driven by substance abuse around pain medications.
Some time this month, Medicare is expected to issue a preliminary decision about whether or not it will cover the expensive new Alzheimer’s drug, Aduhelm, created by Biogen. The drug, which is delivered through infusion, has an estimated cost of about $28,000 a year. If it is approved for treatment, about 1.5 million patients 65 or older will become eligible to receive the drug, resulting in the potential of nearly $100 million a year in new pharmacy costs being covered by federal health insurance policies.
Most drug prices are controlled by federal regulations, although states, when motivated, can negotiate buying certain “drugs” in bulk with other states, such as flu vaccines.
And, there are opportunities available to the R.I. General Assembly to exercise some amount of control over the way that drug policies are implemented in the Rhode Island.
Step therapy
One example of drug policy coming under increasing scrutiny is the process of what is known as Step Therapy. Last year, the R.I. Senate considered legislation, Senate Bill 302, An Act Relating to Insurance – Accident and Sickness Insurance Policies – Step Therapy. It passed the Senate by a roll call vote of 36-0 with two abstentions. The bill was not approved by the R.I. House.
As the R.I. General Assembly begins its 2022 session on Jan.4, it seemed worthwhile to share the testimony of Dr. Jonathan Cahill, a neurologist, in anticipation that the proposed legislation may re-emerge in the coming year.
Thank you for the opportunity to testify in support of Senate Bill 302. I am a Providence resident. I am a neurologist, practicing in Providence for the past 10 years; my subspecialty focus is multiple sclerosis [MS]. I am a member of the Board of Trustees for the Greater New England Chapter of the National Multiple Sclerosis Society and the Secretary of the Rhode Island Neurological Society.
Step therapy policies, by requiring the “failure” of one drug or more before allowing patients access to the initially prescribed medication, interfere with patient-centered decision making.
They are meant to ensure that safe, appropriate, and affordable drugs are provided to patients, but problems arise when step therapy requirements dictate which drugs individuals may access, and when decisions made by patients with their doctors are second-guessed or overturned.
One problem is that the definition of failure of one or another drug is not standard, and the person determining failure is not always clear [the prescribing physician, the patient, the insurance company]. Only treating physicians have the necessary background and understanding of the patient’s circumstances to accurately determine drug treatment failure.
Additionally, some policies do not consider failures of drugs that occurred when the patient was covered by a prior health insurance plan. This is clearly not in the best interests of patients to require them to restart medications that previously were not effective or to which they were intolerant.
Most of my patients live with MS, which is a chronic disabling condition affecting the central nervous system. Since the first drug proven to alter the course of MS was approved by the U.S. Food and Drug Administration [FDA] in 1993, there have been many incredible advances in drug therapy to treat the disease. There are now over 20 FDA-approved disease modifying therapies, and they are giving people with MS more control over the disease and leading to less disability over time.”
More effective than others
Many studies indicate that some of the medications are more effective than others. Furthermore, initiating therapy at diagnosis with the highly effective medications prevents disability more effectively than escalating to more effective therapies in response to disease activity or treatment failure.
The decision about which medication is right for a patient is complicated. Many factors including particulars about the individual’s symptoms and disease course, the available testing, patient preferences, other medical issues, potential risk for side effects, and duration of treatment are all-important factors to consider. This type of consideration is what happens every day in doctors’ offices as physicians and their patients decide on patient-specific treatment plans.
In my experience, too frequently, the step therapy requirements are designed to deny patients access to the newest and most highly effective drugs. Step therapy policies are frequently not supported by medical studies or reflected in the usage of these medications in routine practice.
For example, one of the most highly effective MS medications is frequently not available to patients because of step therapy policies requiring the failure of another drug [or two, or three] first. The policies consider this medication ‘second line,’ although it was studied and gained FDA-approval as a first-line agent.
Step therapy policies rarely can be overturned or appealed by patients or their physicians. In cases where I have appealed on behalf of patients for the prescribed drug and provided a medical rationale for prescribing the drug, most commonly the step therapy policy is not overturned, and the patient is denied access to the medication that the patient and I think would be best based on sound medical research.”
Five examples
I would like to include five examples of step therapy policies I have encountered over the past few years. This is not a complete list, but a representative sample to provide an idea of the circumstances encountered by patients every day throughout Rhode Island:
• I prescribed dextromethorphan/quinidine [Nuedexta] for the treatment of pseudobulbar affect in a patient with amyotrophic lateral sclerosis [ALS)]. Nuedexta is the only FDA-approved medication for this condition. There is no generic formulation available.
The initial denial letter said that because the patient had not “tried and had a poor response to treatment with a tricyclic antidepressant [TCA] or a selective serotonin reuptake inhibitor [SSRI]” the medication was not approved. Neither TCA’s nor SSRI’s were appropriate for treating pseudobulbar affect in this patient.
• I prescribed ocrelizumab [Ocrevus[ as first line therapy for the treatment of relapsing MS, an FDA-approved indication. There is no generic formulation available. Ocrevus is a highly effective medication in MS and was studied as first-line therapy in 73 percent of clinical trial subjects.
The denial indicated a series of “preferred/lower cost drugs that are therapeutically comparable” to Ocrevus, including at least one that had been proven to be inferior to Ocrevus in controlled clinical trials, and several others that have lower rates of relapse reduction than Ocrevus in MS patients.
• I renewed a prescription for carbamazepine [generic formulation] for the treatment of trigeminal neuralgia pain [an FDA-approved indication] in a patient with neurofibromatosis.
Carbamazepine had been recently been moved to a higher tier copay, and the patient, despite having been stable on carbamazepine, needed “to first try other covered medication used to treat [the patient’s] condition in [a lower tier] which includes baclofen.” Baclofen is not FDA-approved for trigeminal neuralgia and was not appropriate for the treatment of this patient.
• Four times so far in 2021, patients with MS who have been stable for more than five years on a specific treatment [interferon-beta 1a – Avonex[ were told by insurance companies that the medication would no longer be approved; and that they were required to switch to a different medication.
For MS patients, it is not advised to switch therapies for patients who are stable, and there is evidence that switching, even to a different drug in the same class, may lead to worsening disease and different or worse side effects.
• Twice in 2021, I have appealed for patients to stay on their current MS treatment [in both cases Tecfidera], because they have already tried and failed two of the medications on the approved list.
The denials of the appeals in both of these cases indicated that the patients had not tried two approved agents already, in clear contradiction to the provided medical records and my own letters of appeal. It seems to me as though the appeal reviewers had not read or chose to ignore my appeals.
Several key points
These examples highlight several key points about step therapy protocols. Step therapy requirements are applied both to branded and generic medications. Step therapy requirements are applied to medications for which there are no same-in-class or generic medications available.
The sequencing of medications in most step therapy protocols is arbitrary, and not supported by clinical trials or other studies. In many cases, less effective or non-FDA-approved medications are required as part of step therapy protocols.
And finally, the appeals process does not give patients or their providers adequate voice. In my experience, step therapy policies are becoming more prevalent and more restrictive over the past few years. In response, 23 states have enacted legislation to allow for individuals to easily apply for exemptions to step therapy policies. I urge Rhode Island to join those states and pass Senate Bill 302.
In summary, the step therapy requirements for patients to fail “preferred” agents are not supported by science, are not helpful for patients, create undue administrative burden, and reduce access to highly effective therapies. Allowing individuals, especially those with chronic diseases such as MS, access to step therapy protocol exceptions will insure that Rhode Island residents receive the best care possible. Thank you.
Dr. Jonathan Cahill is an Associate Professor of Neurology at the Warren Alpert Medical School and a staff neurologist at Rhode Island Hospital.