Nipping Alzheimer’s disease in the blood
MindImmune, a drug discovery firm at URI, is poised to move into clinical trials, pioneering a breakthrough approach to preventing Alzheimer’s disease, focused on blocking aberrant immune cells in the blood from entering the brain
KINGSTON – MindImmune, a drug discovery firm, working in collaboration with the George & Anne Ryan Institute for Neuroscience at URI, recently completed a $12.4 million Series A round of financing.
The firm is being backed by a syndicate of investors, including Dolby Family Ventures, Pfizer Ventures, the Alzheimer’s Drug Discovery Foundation, Trend Venture, and Right Hill Ventures, a division of the Slater Technology Fund.
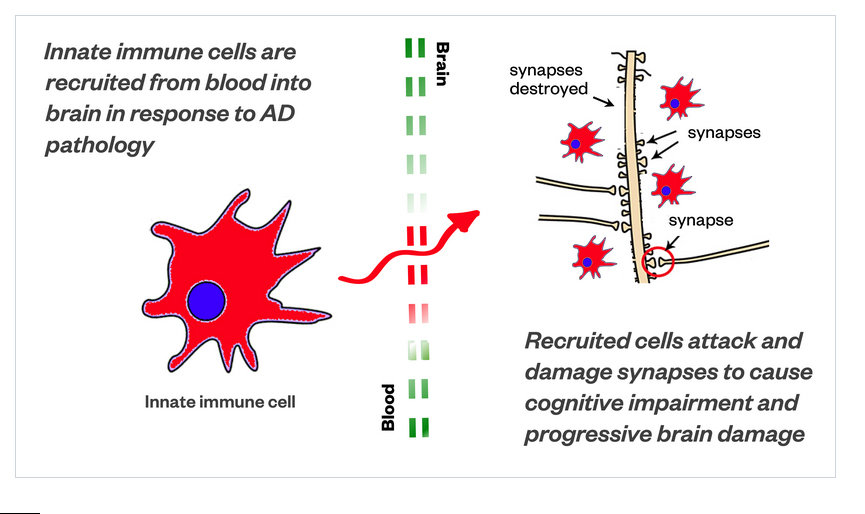
The scientists at MindImmune have discovered an innate immune cell from the blood that enters the brain and causes the synaptic damage at the core of Alzheimer’s symptoms and progression, according to the research data. In response, MindImmune is developing a novel antibody therapeutic, a biologic, that ameliorates the synaptic damage, simply by preventing these immune cells from entering the brain.
Stevin Zorn, Ph.D., president and CEO of MindImmune Therapeutics, Inc., recently spoke at length with ConvergenceRI, describing in detail the exciting new discoveries by MindImmune.
Here is the ConvergenceRI interview with Zorn, who, along with his colleagues, have been featured in a number of stories by ConvergenceRI.
ConvergenceRI: Can you tell me the latest news from MindImmune?
ZORN: We had opened up our first Series A round in 2019, and we closed it this year.
This work is specifically related to Alzheimer’s disease, [focused] on peripheral immune cells as a way of attacking immune cell damage in the brain.
We’ve made a lot of progress on that over the last couple of years. And, it is that progress that has been rewarded in this investment. We’re very excited about it.
ConvergenceRI: Can you tell me more about the science?
ZORN: I don’t know if you have looked at our website recently. We just updated it on June 13. On the website, we put, for the first time, a summary of the science; there are a couple of diagrams, where we have a stylized drawing of innate immune cell.
One of the things that we discovered is that those particular type of innate immune cells are recruited into the brain, in times of inflammatory brain disease. And, the cells have a way of getting through the blood brain barrier. Which, in drug discovery, getting a drug through the blood brain barrier is the biggest challenge.
Then, once it gets through the blood brain barrier, getting it to the target in the brain is the second big challenge.
These cells have a way of getting into the brain as immune cells, but when they get in there, something bad happens.
There is a picture in the diagram [see first image], we’ve drawn it to look a little like a Pac-Man. They are called phagocytic cells, which means they chew up things. They are supposed to chew up debris – bad proteins, and foreign invaders, like bacteria or viruses.
They are also supposed to help repair things. But what these cells are actually doing is attacking synapses, the neuronal connections of brain cells.
These [aberrant Pac-Man] immune cells attack and damage the very fundamental units of our brain’s ability to communicate with itself. And, that causes progressive brain damage.
One of the exciting features about what we discovered is that if we keep these cells out of the brain, we can stop the pathology that is causing the disruption of the neurons – and then the neurons can actually repair themselves – all by stopping these aberrant cells from getting into the brain.
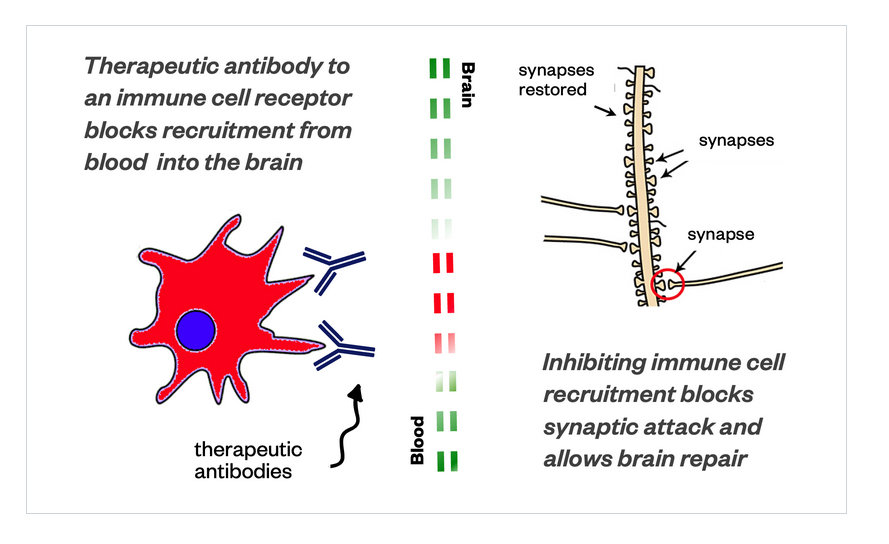
And, we also discovered a target, a unique molecular target, not in the cells, but in the blood, and we discovered an antibody that neutralizes this target. By neutralizing it, these cells are not able to figure out how to get into the brain anymore.
And, when they don’t get into the brain, the synapses are repaired. By inhibiting the recruitment of these cells into the brain – [you are] basically blocking the synaptic attack by the immune system, it allows the brain to repair itself. And, that has a very, very direct link to Alzheimer’s disease.
If you look at the drawing on the website, I think it is pretty clear what these PAC-Man immune cells are doing.
CnnvergenceRI: MindImmune has been taking a different scientific approach, looking at neuro-inflammation.
ZORN: One of the reasons why this approach is interesting, is probably 15-20 years ago, people didn’t believe that immune cells from the periphery, from the blood, had any role to play with central nervous system [CNS] diseases. They thought that the immune system in the brain was largely related to immune cells that were already there.
Our data suggests these immune cells from the blood are the perpetrators of these neuro-inflammation diseases, particularly Alzheimer’s and if we stop them [from entering] the brain, we can stop the disease process in its tracks – and restore brain function. We have lots of data to support that [conclusion].
ConvergenceRI: Where is this data coming from? Are you working with blood samples? Are you working with mice? How are the experiments being conducted?
ZORN: The research largely has been guided by genetics, looking at Alzheimer’s disease genetic material from patients, which has enabled the construction of transgenic animals that mimic parts of the disease process.
And, we can look at transgenic Alzheimer’s disease-related animals. We did the human genetics; we also conducted the genetics studies in these Alzheimer model animals, and we found similar genetics that pointed us toward these immune cells.
Guided by both the human and the animal model genetics, we were able to use the animal models in which to track the system and to look at the way these cells damage neuro-connections.
That enabled us to probe what happens if we prevent these cells from getting into the brain. And. when we did that, we were very excited to find that we could stop, in very dramatic fashion, the neural damage that was occurring, and that neural damage very quickly repaired itself. [Obviously, if the brain is dead, you can’t repair it.]
But, if you work with the cells in the stage where they can be repaired by stopping the synaptic attack, they can repair themselves and get better.
ConvergenceRI: Having developed this target, basically you can attach it to the cell that is part of the immune system in the blood, and prevent it from getting into the brain, which prevents it from doing damage, How does that then translate into treatment?
ZORN: We have discovered a target on the cell, the receptor on the cell that interacts with parts oft the blood brain barrier – that recognizes it and helps it to transfer through the blood brain barrier. We created an antibody to this target on the cell, a biologic, and we were able to inject the biologic into these transgenic animals, and get a therapeutic response. What we are working on now is building an antibody biologic that can be administered as a drug.
ConvergenceRI: Wow! And, and how far away are you from clinical trials?
ZORN: We are now looking to identify the candidate that we want to take into clinical trials. And, as part of the program, we have also developed a biomarker.
One of the things that have been very difficult in this field, Richard, is that there are really no good biomarkers for neuro-inflammation. And, we think we have found one.
And, we think that we found one that tracks very nicely with our ability to measure the recruitment of these immune cells into the brain. We can image them.
We’ve been able to do this in animal studies. We can see these types of cells around human Alzheimer’s pathology. We are actually getting ready to do a clinical study to validate our biomarker in the coming months, this year.
We joined forces with the ADDF to fund that clinical study. That is part of our Series A financing, as part of the syndicate.
ConvergenceRI: Where will the clinical study take place for the biomarker?
ZORN: We are looking at a couple of sites. Mostly, how we work is we have this very nice collaboration, a public-private partnership, at the University of Rhode Island, with the George & Anne Ryan Institute for Neuroscience.
We have a small laboratory here; we have experiments ongoing, but for most of the work we do, we contract through CROs [contract research organizations].
And, we contracted with a couple of clinical CROs to execute these clinical studies.
We are working with them to recruit our subjects. They have some defined sites, so I’m not exactly sure where the first patient goes for the biomarker study.
ConvergenceRI: It all sounds very exciting. What has been the response? I would guess that lots of people in the field are watching what you are doing very carefully.
ZORN: Yes.
ConvergenceRI: As much as you have been focused on a biomarker for neuro- inflammation for Alzheimer’s disease, is there any potential for any crossover with what’s happening with COVID? Or, is that too far away from your focus?
ZORN: You know, that is of personal interest, but it is far away from our focus. Clearly COVID is an immunological disease. We believe Alzheimer’s, and a number of neurodegenerative conditions, are also related to neuro-immunlogical disease.
One of the ways we are thinking about this is that we discovered something that is a fundamental biology, related to chronic inflammation conditions. We haven’t really thought about Alzheimer’s disease as a standard chronic inflammatory condition, But we are finding that mechanisms across the chronic inflammatory conditions are similar.
Before, few thought about Alzhemier’s as a chronic inflammatory condition, it’s a new way of thinking. And, to attack the process that causes those inflammatory conditions, in particular, by attacking the peripheral immune cells, [is a novel approach].
The question about the biomarker that is important, obviously, is that for the biomarker study, you want to be able to measure something that predicts an outcome, long before we have a long-term expensive clinical study.
The other thing is that given the importance of neuro-inflammation in underlying brain diseases as a cause – or a very significant contributor, when you can be able to measure that, and we think that our biomarker has the potential to do both – to serve as a biomarker as part of outcomes for our clinical studies, but also as ubiquitous biomarker of neuro-inflammation of the brain. Which is really needed in this field. We are very excited about this, Richard,